Enzyme Involved In Dna Replication
As discussed in Chapter 3, DNA replication is a semiconservative process in which each parental strand serves every bit a template for the synthesis of a new complementary daughter strand. The central enzyme involved is DNA polymerase, which catalyzes the joining of deoxyribonucleoside five′-triphosphates (dNTPs) to form the growing DNA chain. However, DNA replication is much more complex than a single enzymatic reaction. Other proteins are involved, and proofreading mechanisms are required to ensure that the accuracy of replication is compatible with the low frequency of errors that is needed for jail cell reproduction. Additional proteins and specific DNA sequences are as well needed both to initiate replication and to copy the ends of eukaryotic chromosomes.
DNA Polymerases
DNA polymerase was commencement identified in lysates of East. coli by Arthur Kornberg in 1956. The ability of this enzyme to accurately copy a DNA template provided a biochemical footing for the manner of DNA replication that was initially proposed by Watson and Crick, then its isolation represented a landmark discovery in molecular biology. Ironically, however, this outset DNA polymerase to be identified (now called DNA polymerase I) is not the major enzyme responsible for E. coli Dna replication. Instead, information technology is now clear that both prokaryotic and eukaryotic cells contain several different DNA polymerases that play distinct roles in the replication and repair of Dna.
The multiplicity of Deoxyribonucleic acid polymerases was first revealed by the isolation of a mutant strain of Eastward. coli that was deficient in polymerase I (Figure 5.ane). Cultures of E. coli were treated with a chemical (a mutagen) that induces a high frequency of mutations, and individual bacterial colonies were isolated and screened to identify a mutant strain lacking polymerase I. Assay of a few k colonies led to the isolation of the desired mutant, which was about totally lacking in polymerase I action. Surprisingly, the mutant bacteria grew ordinarily, leading to the decision that polymerase I is not required for Deoxyribonucleic acid replication. On the other paw, the mutant leaner were extremely sensitive to agents that damage DNA (east.g., ultraviolet low-cal), suggesting that polymerase I is involved primarily in the repair of Deoxyribonucleic acid damage rather than in Dna replication per se.

Figure five.i
Isolation of a mutant deficient in polymerase I. A civilisation of Due east. coli was treated with a chemical mutagen, and individual bacterial colonies were isolated by growth on semisolid medium. Several grand colonies were and so cultured and screened to place (more...)
The conclusion that polymerase I is not required for replication implied that East. coli must contain other Deoxyribonucleic acid polymerases, and subsequent experiments led to the identification of two such enzymes, now called DNA polymerases II and 3. The potential roles of these enzymes were investigated by the isolation of appropriate mutants. Strains of Due east. coli with mutations in polymerase 2 were constitute to grow and otherwise carry normally, so the role of this enzyme in the cell is unknown. Temperature-sensitive polymerase III mutants, notwithstanding, were unable to replicate their Dna at high temperature, and subsequent studies accept confirmed that polymerase Three is the major replicative enzyme in E. coli.
Information technology is now known that, in improver to polymerase III, polymerase I is besides required for replication of E. coli DNA. The original polymerase I mutant was non completely defective in that enzyme, and later experiments showed that the residue polymerase I activeness in this strain plays a key office in the replication process. The replication of E. coli DNA thus involves two distinct Deoxyribonucleic acid polymerases, the specific roles of which are discussed beneath.
Eukaryotic cells contain five Dna polymerases: α, β, γ, δ, and ε. Polymerase γ is located in mitochondria and is responsible for replication of mitochondrial Deoxyribonucleic acid. The other four enzymes are located in the nucleus and are therefore candidates for involvement in nuclear Dna replication. Polymerases α, δ, and ε are near active in dividing cells, suggesting that they function in replication. In contrast, polymerase β is active in nondividing and dividing cells, suggesting that it may office primarily in the repair of DNA damage.
2 types of experiments have provided further evidence addressing the roles of polymerases α, δ, and ε in DNA replication. First, replication of the DNAs of some brute viruses, such as SV40, tin be studied in jail cell-free extracts. The power to study replication in vitro has allowed straight identification of the enzymes involved, and analysis of such cell-free systems has shown that polymerases α and δ are required for SV40 Dna replication. Second, polymerases α, δ, and ε are found in yeasts as well as in mammalian cells, enabling the utilize of the powerful approaches of yeast genetics (see Chapter 3) to examination their biological roles directly. Such studies indicate that yeast mutants defective whatsoever of these three DNA polymerases are unable to proliferate, implying a critical role for polymerase ε besides as for α and δ. However, further studies take shown that the essential function of polymerase ε in yeast does not require its activity as a replicative DNA polymerase. Thus, polymerases α and δ appear to be sufficient for Dna replication both in cell-free systems and in yeast, so the role of polymerase ε remains to be established.
All known Deoxyribonucleic acid polymerases share two primal properties that acquit critical implications for DNA replication (Figure 5.two). First, all polymerases synthesize Dna merely in the 5′ to 3′ management, calculation a dNTP to the 3′ hydroxyl grouping of a growing chain. Second, Dna polymerases can add a new deoxyribonucleotide only to a preformed primer strand that is hydrogen-bonded to the template; they are non able to initiate Dna synthesis de novo by catalyzing the polymerization of free dNTPs. In this respect, Deoxyribonucleic acid polymerases differ from RNA polymerases, which can initiate the synthesis of a new strand of RNA in the absence of a primer. As discussed afterwards in this chapter, these properties of DNA polymerases appear critical for maintaining the loftier fidelity of Dna replication that is required for cell reproduction.
Figure v.2
The reaction catalyzed past Dna polymerase. All known Dna polymerases add together a deoxyribonucleoside five′-triphosphate to the 3′ hydroxyl group of a growing DNA chain (the primer strand).
The Replication Fork
DNA molecules in the procedure of replication were first analyzed by John Cairns in experiments in which E. coli were grown in the presence of radioactive thymidine, which allowed subsequent visualization of newly replicated Deoxyribonucleic acid by autoradiography (Figure five.three). In some cases, complete circular molecules in the procedure of replicating could be observed. These Dna molecules independent 2 replication forks, representing the regions of active DNA synthesis. At each fork the parental strands of Dna separated and two new girl strands were synthesized.

Effigy v.3
Replication of E. coli Deoxyribonucleic acid. (A) An autoradiograph showing bacteria that were grown in [3H]thymidine for two generations to label the Deoxyribonucleic acid, which was then extracted and visualized by exposure to photographic movie. (B) This schematic illustrates the two (more...)
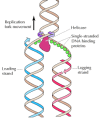
The synthesis of new Deoxyribonucleic acid strands complementary to both strands of the parental molecule posed an of import problem to understanding the biochemistry of Deoxyribonucleic acid replication. Since the two strands of double-helical Deoxyribonucleic acid run in opposite (antiparallel) directions, continuous synthesis of two new strands at the replication fork would require that one strand be synthesized in the 5′ to 3′ management while the other is synthesized in the opposite (3′ to 5′) management. Simply Deoxyribonucleic acid polymerase catalyzes the polymerization of dNTPs only in the 5′ to 3′ direction. How, then, can the other progeny strand of DNA be synthesized?
This enigma was resolved past experiments showing that just one strand of DNA is synthesized in a continuous way in the direction of overall Dna replication; the other is formed from small-scale, discontinuous pieces of Deoxyribonucleic acid that are synthesized astern with respect to the direction of movement of the replication fork (Figure v.4). These minor pieces of newly synthesized DNA (called Okazaki fragments after their discoverer) are joined by the activity of DNA ligase, forming an intact new DNA strand. The continuously synthesized strand is chosen the leading strand, since its elongation in the direction of replication fork movement exposes the template used for the synthesis of Okazaki fragments (the lagging strand).

Figure 5.iv
Synthesis of leading and lagging strands of DNA. The leading strand is synthesized continuously in the management of replication fork motility. The lagging strand is synthesized in pocket-size pieces (Okazaki fragments) backward from the overall management of (more than...)
Although the discovery of discontinuous synthesis of the lagging strand provided a mechanism for the elongation of both strands of Dna at the replication fork, information technology raised some other question: Since Dna polymerase requires a primer and cannot initiate synthesis de novo, how is the synthesis of Okazaki fragments initiated? The answer is that curt fragments of RNA serve as primers for Deoxyribonucleic acid replication (Figure 5.five). In contrast to Dna synthesis, the synthesis of RNA can initiate de novo, and an enzyme called primase synthesizes short fragments of RNA (e.chiliad., 3 to ten nucleotides long) complementary to the lagging strand template at the replication fork. Okazaki fragments are then synthesized via extension of these RNA primers by Dna polymerase. An important upshot of such RNA priming is that newly synthesized Okazaki fragments contain an RNA-Deoxyribonucleic acid joint, the discovery of which provided disquisitional evidence for the office of RNA primers in Deoxyribonucleic acid replication.

Figure 5.five
Initiation of Okazaki fragments with RNA primers. Short fragments of RNA serve as primers that tin can be extended by DNA polymerase.
To form a continuous lagging strand of Deoxyribonucleic acid, the RNA primers must somewhen be removed from the Okazaki fragments and replaced with DNA. In East. coli, RNA primers are removed by the combined action of RNase H, an enzyme that degrades the RNA strand of RNA-Dna hybrids, and polymerase I. This is the attribute of E. coli DNA replication in which polymerase I plays a critical role. In add-on to its Deoxyribonucleic acid polymerase activity, polymerase I acts equally an exonuclease that tin hydrolyze Dna (or RNA) in either the 3′ to 5′ or 5′ to 3′ management. The action of polymerase I as a v′ to 3′ exonuclease removes ribonucleotides from the 5′ ends of Okazaki fragments, allowing them to be replaced with deoxyribonucleotides to yield fragments consisting entirely of DNA (Figure 5.half-dozen). In eukaryotic cells, other exonucleases take the identify of E. coli polymerase I in removing primers, and the gaps between Okazaki fragments are filled by the action of polymerase δ. As in prokaryotes, these DNA fragments can and then be joined by Dna ligase.
Figure 5.6
Removal of RNA primers and joining of Okazaki fragments. Because of its 5′ to iii′ exonuclease activeness, DNA polymerase I removes RNA primers and fills the gaps betwixt Okazaki fragments with DNA. The resultant DNA fragments can then be (more...)
The different DNA polymerases thus play distinct roles at the replication fork (Effigy 5.7). In prokaryotic cells, polymerase III is the major replicative polymerase, functioning in the synthesis both of the leading strand of DNA and of Okazaki fragments by the extension of RNA primers. Polymerase I then removes RNA primers and fills the gaps between Okazaki fragments. In eukaryotic cells, however, 2 Dna polymerases are required to do what in E. coli is achieved by polymerase 3 alone. Polymerase α is institute in a complex with primase, and it appears to role in conjunction with primase to synthesize curt RNA-Deoxyribonucleic acid fragments during lagging strand synthesis. Polymerase δ tin so synthesize both the leading and lagging strands, acting to extend the RNA-DNA primers initially synthesized by the polymerase α-primase circuitous. In addition, polymerase δ can take the place of Eastward. coli polymerase I in filling the gaps between Okazaki fragments post-obit primer removal.

Figure 5.seven
Roles of DNA polymerases in East. coli and mammalian cells. The leading strand is synthesized past polymerase Iii (pol III) in E. coli and by polymerase δ (politician δ) in mammalian cells. In E. coli, lagging strand synthesis is initiated by primase, (more...)
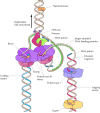
Non only polymerases and primase but likewise a number of other proteins deed at the replication fork. These additional proteins accept been identified both by the assay of Eastward. coli mutants lacking in DNA replication and past the purification of the mammalian proteins required for in vitro replication of SV40 DNA. One class of proteins required for replication binds to DNA polymerases, increasing the activeness of the polymerases and causing them to remain bound to the template DNA so that they keep synthesis of a new Dna strand. Both E. coli polymerase Iii and eukaryotic polymerase δ are associated with two types of accessory proteins (sliding-clamp proteins and clamp-loading proteins) that load the polymerase onto the primer and maintain its stable association with the template (Effigy five.8). The clamp-loading proteins (chosen the γ complex in Due east. coli and replication factor C [RFC] in eukaryotes) specifically recognize and bind DNA at the junction between the primer and template. The sliding-clamp proteins (β protein in E. coli and proliferating prison cell nuclear antigen [PCNA] in eukaryotes) demark adjacent to the clamp-loading proteins, forming a ring around the template Deoxyribonucleic acid. The clench proteins then load the Dna polymerase onto Dna at the primer-template junction. The ring formed past the sliding clamp maintains the association of the polymerase with its template as replication proceeds, allowing the uninterrupted synthesis of many thousands of nucleotides of DNA.

Figure 5.8
Polymerase accessory proteins. (A) The clamp-loading protein (RFC in mammalian cells) binds DNA at the junction between primer and template. The sliding-clamp poly peptide (PCNA in mammalian cells) binds side by side to the RFC, and DNA polymerase and then binds to (more than...)
Other proteins unwind the template DNA and stabilize single-stranded regions (Effigy v.9). Helicases are enzymes that catalyze the unwinding of parental Dna, coupled to the hydrolysis of ATP, ahead of the replication fork. Single-stranded DNA-bounden proteins (due east.g., eukaryotic replication factor A [RFA]) then stabilize the unwound template Deoxyribonucleic acid, keeping it in an extended unmarried-stranded state so that it tin exist copied by the polymerase.
Effigy 5.9
Action of helicases and unmarried-stranded DNA-bounden proteins. Helicases unwind the 2 strands of parental DNA ahead of the replication fork. The unwound DNA strands are then stabilized by single-stranded Deoxyribonucleic acid-bounden proteins then that they can serve equally (more...)
As the strands of parental Dna unwind, the DNA alee of the replication fork is forced to rotate. Unchecked, this rotation would cause circular Dna molecules (such as SV40 DNA or the E. coli chromosome) to become twisted effectually themselves, eventually blocking replication (Figure 5.10). This trouble is solved by topoisomerases, enzymes that catalyze the reversible breakage and rejoining of Deoxyribonucleic acid strands. In that location are two types of these enzymes: Blazon I topoisomerases break just one strand of Dna; type II topoisomerases introduce simultaneous breaks in both strands. The breaks introduced by type I and type II topoisomerases serve as "swivels" that allow the ii strands of template Dna to rotate freely effectually each other so that replication tin proceed without twisting the Dna ahead of the fork (see Figure 5.10). Although eukaryotic chromosomes are composed of linear rather than round Dna molecules, their replication also requires topoisomerases; otherwise, the consummate chromosomes would have to rotate continually during DNA synthesis.

Figure v.10
Action of topoisomerases during DNA replication. (A) Equally the 2 strands of template Deoxyribonucleic acid unwind, the Dna ahead of the replication fork is forced to rotate in the reverse direction, causing round molecules to get twisted around themselves. (B) This (more...)
Blazon 2 topoisomerase is needed not just to unwind Dna but also to unravel newly replicated circular DNA molecules that go interwined with each other. In eukaryotic cells, topoisomerase Two appears to exist involved in mitotic chromosome condensation. In add-on, studies of yeast mutants, also as experiments in Drosophila and mammalian cells, signal that topoisomerase Ii is required for the separation of daughter chromatids at mitosis, suggesting that it functions to untangle newly replicated loops of DNA in the chromosomes of eukaryotes.
The enzymes involved in DNA replication human activity in a coordinated way to synthesize both leading and lagging strands of DNA simultaneously at the replication fork (Figure five.11). This task is accomplished past the formation of dimers of the replicative Deoxyribonucleic acid polymerases (polymerase Iii in Due east. coli or polymerase δ in eukaryotes), each with its appropriate accompaniment proteins. 1 molecule of polymerase then acts in synthesis of the leading strand while the other acts in synthesis of the lagging strand. The lagging strand template is thought to form a loop at the replication fork then that the polymerase subunit engaged in lagging strand synthesis moves in the same overall direction as the other subunit, which is synthesizing the leading strand.
Effigy v.xi
Model of the E. coli replication fork. Helicase, primase, and ii molecules of Deoxyribonucleic acid polymerase Three acquit out coordinated synthesis of both the leading and lagging strands of DNA. The lagging strand template is folded then that the polymerase responsible (more...)
The Fidelity of Replication
The accuracy of Dna replication is critical to cell reproduction, and estimates of mutation rates for a variety of genes signal that the frequency of errors during replication corresponds to only one incorrect base per 109 to 1010 nucleotides incorporated. This error frequency is much lower than would be predicted but on the basis of complementary base pairing. In particular, the standard configurations of nucleic acrid bases are in equilibrium with rare culling conformations (tautomeric forms) that hydrogen-bail with the wrong partner (e.thou., G with T) with a frequency of near one incorrect base of operations per 104 (Figure five.12). The much higher degree of fidelity actually accomplished results largely from the activities of Dna polymerase.

Figure 5.12
Mismatching between rare configurations of nucleic acid bases. In its normal configuration, guanine (G) specifically forms complementary hydrogen bonds (dashed lines) with cytosine (C). However, G occasionally assumes a rare configuration (tautomeric (more...)
One mechanism by which DNA polymerase increases the fidelity of replication is past helping to select the right base for insertion into newly synthesized Deoxyribonucleic acid. The polymerase does not simply catalyze incorporation of any nucleotide is hydrogen-bonded to the template strand. Instead, it actively discriminates against incorporation of a mismatched base of operations, presumably past adapting to the conformation of a correct base of operations pair. The molecular mechanisms responsible for the ability of DNA polymerases to select against incorrect bases are non yet entirely understood, but this selectivity appears to increase the accurateness of replication about a hundredfold, reducing the expected mistake frequency from 10-iv to approximately 10-six.
The other major mechanism responsible for the accuracy of Dna replication is the proofreading activity of DNA polymerase. As already noted, E. coli polymerase I has 3′ to 5′ as well every bit five′ to 3′ exonuclease activity. The 5′ to 3′ exonuclease operates in the direction of Dna synthesis and helps remove RNA primers from Okazaki fragments. The three′ to v′ exonuclease operates in the reverse management of DNA synthesis, and participates in proofreading newly synthesized DNA (Figure 5.13). Proofreading is constructive considering DNA polymerase requires a primer and is non able to initiate synthesis de novo. Primers that are hydrogen-bonded to the template are preferentially used, so when an incorrect base of operations is incorporated, it is likely to exist removed by the 3′ to 5′ exonuclease activity rather than being used to proceed synthesis. Such three′ to 5′ exonuclease activities are besides associated with E. coli polymerase Iii and eukaryotic polymerases δ and ε. The iii′ to 5′ exonucleases of these polymerases selectively excise mismatched bases that have been incorporated at the end of a growing DNA chain, thereby increasing the accuracy of replication past a hundred- to a thousandfold.
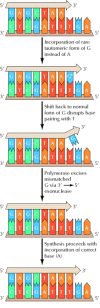
Figure v.13
Proofreading by Dna polymerase. A rare tautomeric form of Thou (G*) is incorporated in identify of A as a upshot of mispairing with T on the template strand. The subsequent shift of Grand back to its normal form disrupts this base pairing, so the 3′ terminal (more...)
The importance of proofreading may explain the fact that DNA polymerases require primers and catalyze the growth of Dna strands only in the 5′ to 3′ direction. When DNA is synthesized in the 5′ to three′ direction, the energy required for polymerization is derived from hydrolysis of the 5′ triphosphate group of a free dNTP every bit it is added to the 3′ hydroxyl grouping of the growing chain (run across Figure 5.two). If Dna were to be extended in the 3′ to v′ management, the energy of polymerization would instead have to be derived from hydrolysis of the 5′ triphosphate grouping of the terminal nucleotide already incorporated into DNA. This would eliminate the possibility of proofreading, because removal of a mismatched terminal nucleotide would also remove the 5′ triphosphate group needed every bit an energy source for farther concatenation elongation. Thus, although the ability of Dna polymerase to extend a primer only in the five′ to three′ management appears to make replication a complicated procedure, information technology is necessary for ensuring accurate duplication of the genetic material.
Combined with the ability to discriminate confronting the insertion of mismatched bases, the proofreading activity of Deoxyribonucleic acid polymerases is sufficient to reduce the error frequency of replication to virtually one mismatched base per 109. Boosted mechanisms (discussed in the section "DNA Repair") act to remove mismatched bases that have been incorporated into newly synthesized Deoxyribonucleic acid, further ensuring correct replication of the genetic information.
Origins and the Initiation of Replication
The replication of both prokaryotic and eukaryotic DNAs starts at a unique sequence chosen the origin of replication, which serves as a specific binding site for proteins that initiate the replication process. The first origin to be defined was that of E. coli, in which genetic analysis indicated that replication always begins at a unique site on the bacterial chromosome. The E. coli origin has since been studied in detail and found to consist of 245 base of operations pairs of DNA, elements of which serve equally binding sites for proteins required to initiate Dna replication (Figure 5.14). The cardinal step is the bounden of an initiator protein to specific DNA sequences within the origin. The initiator protein begins to unwind the origin DNA and recruits the other proteins involved in DNA synthesis. Helicase and unmarried-stranded Deoxyribonucleic acid-binding proteins then act to continue unwinding and exposing the template Dna, and primase initiates the synthesis of leading strands. Two replication forks are formed and move in opposite directions forth the circular E. coli chromosome.

Figure 5.fourteen
Origin of replication in E. coli. Replication initiates at a unique site on the Eastward. coli chromosome, designated the origin (ori). The offset event is the binding of an initiator protein to ori Deoxyribonucleic acid, which leads to partial unwinding of the template. The DNA (more...)
The origins of replication of several animal viruses, such equally SV40, have been studied as models for the initiation of DNA synthesis in eukaryotes. SV40 has a single origin of replication (consisting of 64 base pairs) that functions both in infected cells and in jail cell-free systems. Replication is initiated by a virus-encoded protein (called T antigen) that binds to the origin and also acts every bit a helicase. A single-stranded Deoxyribonucleic acid-binding protein is required to stabilize the unwound template, and the Dna polymerase α-primase complex then initiates DNA synthesis.
Although single origins are sufficient to direct the replication of bacte-rial and viral genomes, multiple origins are needed to replicate the much larger genomes of eukaryotic cells within a reasonable menstruation of time. For instance, the entire genome of Due east. coli (iv × 106 base pairs) is replicated from a unmarried origin in approximately 30 minutes. If mammalian genomes (3 × 109 base pairs) were replicated from a single origin at the same rate, DNA replication would require about 3 weeks (30,000 minutes). The problem is further exacerbated by the fact that the charge per unit of DNA replication in mammalian cells is actually almost tenfold lower than in E. coli, possibly as a result of the packaging of eukaryotic DNA in chromatin. Nonetheless, the genomes of mammalian cells are typically replicated within a few hours, necessitating the utilize of thousands of replication origins.
The presence of multiple replication origins in eukaryotic cells was first demonstrated by the exposure of cultured mammalian cells to radioactive thymidine for unlike fourth dimension intervals, followed by autoradiography to detect newly synthesized Deoxyribonucleic acid. The results of such studies indicated that DNA synthesis is initiated at multiple sites, from which it then gain in both directions forth the chromosome (Figure v.15). The replication origins in mammalian cells are spaced at intervals of approximately 50 to 300 kb; thus the human genome has about xxx,000 origins of replication. The genomes of simpler eukaryotes besides accept multiple origins; for example, replication in yeasts initiates at origins separated by intervals of approximately 40 kb.
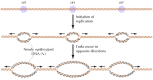
Figure v.15
Replication origins in eukaryotic chromosomes. Replication initiates at multiple origins (ori), each of which produces two replication forks.
The origins of replication of eukaryotic chromosomes have been studied best in yeasts, in which they accept been identified as sequences that tin support the replication of plasmids in transformed cells (Figure 5.16). This has provided a functional assay for these sequences, and several such elements (called autonomously replicating sequences, or ARSs) have been isolated. Their role every bit origins of replication has been verified by direct biochemical analysis, not simply in plasmids but besides in yeast chromosomal Dna.
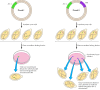
Figure 5.16
Identification of origins of replication in yeast. Both plasmids I and Two contain a selectable marker gene (LEU 2) that allows transformed cells to grow on medium lacking leucine. Only plasmid Two, however, contains an origin of replication (ARS). The (more...)
Functional ARS elements span most 100 base pairs, including an xi-base-pair core sequence mutual to many dissimilar ARSs (Effigy 5.17). This core sequence is essential for ARS office and has been establish to exist the bounden site of a protein circuitous (called the origin replication complex, or ORC) that is required for initiation of Deoxyribonucleic acid replication at yeast origins. The ORC complex appears to recruit other proteins (including DNA helicases) to the origin, leading to the initiation of replication. The mechanism of initiation of Deoxyribonucleic acid replication in yeasts thus appears similar to that in prokaryotes and eukaryotic viruses; that is, an initiator protein specifically binds to origin sequences.

Effigy 5.17
A yeast ARS element. The chemical element contains an xi-base-pair ARS consensus sequence (ACS), which is the specific bounden site of the origin replication circuitous (ORC). Three additional elements (B1, B2, and B3) are individually non essential just together (more...)
In contrast to the well-defined ARS elements in yeasts, much less is known nigh the nature of replication origins in more complex eukaryotes. Nonetheless, recent experiments take shown that specific origin sequences are required for initiation of DNA replication in mammalian cells. In add-on, proteins related to the yeast ORC proteins take been identified in a variety of eukaryotes, including Drosophila, C. elegans, Arabidopsis, and humans, and shown to exist essential for DNA replication. It thus appears likely that the bones mechanism used to initiate DNA replication is conserved in eukaryotic cells.
Telomeres and Telomerase: Replicating the Ends of Chromosomes
Because Dna polymerases extend primers only in the v′ to 3′ direction, they are unable to copy the extreme 5′ ends of linear DNA molecules. Consequently, special mechanisms are required to replicate the terminal sequences of the linear chromosomes of eukaryotic cells. These sequences (telomeres) consist of tandem repeats of unproblematic-sequence DNA (see Affiliate four). They are replicated by the action of a unique enzyme called telomerase, which is able to maintain telomeres by catalyzing their synthesis in the absenteeism of a DNA template.
Telomerase is a reverse transcriptase, one of a class of DNA polymerases, first discovered in retroviruses (meet Chapter iii), that synthesize Dna from an RNA template. Importantly, telomerase carries its own template RNA, which is complementary to the telomere repeat sequences, as part of the enzyme circuitous. The use of this RNA every bit a template allows telomerase to generate multiple copies of the telomeric repeat sequences, thereby maintaining telomeres in the absenteeism of a conventional Dna template to straight their synthesis.
The machinery of telomerase action was initially elucidated in 1985 by Carol Greider and Elizabeth Blackburn during studies of the protozoan Tetrahymena (Figure 5.18). The Tetrahymena telomerase is complexed to a 159-nucleotide-long RNA that includes the sequence three′-AACCCCAAC-5′. This sequence is complementary to the Tetrahymena telomeric echo (v′-TTGGGG-3′) and serves every bit the template for the synthesis of telomeric Dna. The use of this RNA as a template allows telomerase to extend the 3′ end of chromosomal Deoxyribonucleic acid by ane repeat unit beyond its original length. The complementary strand can then be synthesized by the polymerase α-primase complex using conventional RNA priming. Removal of the RNA primer leaves an overhanging 3′ cease of chromosomal DNA, which can form loops at the ends of eukaryotic chromosomes (see Effigy 4.19).
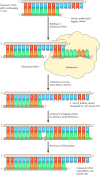
Figure five.18
Action of telomerase. Telomeric Dna is a elementary echo sequence with an overhanging three′ end on the newly synthesized leading strand. Telomerase carries its ain RNA molecule, which is complementary to telomeric DNA, as part of the enzyme complex. (more...)
Telomerase has been identified in a variety of eukaryotes, and genes encoding telomerase RNAs take been cloned from Tetrahymena, yeasts, mice, and humans. In each case, the telomerase RNA contains sequences complementary to the telomeric repeat sequence of that organism (see Tabular array 4.3). Moreover, the introduction of mutant telomerase RNA genes into yeasts has been shown to result in corresponding alterations of the chromosomal telomeric repeat sequences, directly demonstrating the function of telomerase in maintaining the termini of eukaryotic chromosomes.

Enzyme Involved In Dna Replication,
Source: https://www.ncbi.nlm.nih.gov/books/NBK9940/
Posted by: oconnellsilth1993.blogspot.com


0 Response to "Enzyme Involved In Dna Replication"
Post a Comment